Leveraging genetically-encoded heterogeneity to understand TANDs and seizures in novel models of TSC
NIH NINDS 1R01NS134491
The long-term goal of this project is to understand the mechanisms that underlie the neurological consequences of Tuberous Sclerosis Complex (TSC). TSC is a profoundly complex genetic disorder caused by mutations in either the TSC1 or TSC2 gene and characterized by benign tumor growth in multiple systems in the body. TSC is associated with epilepsy in up to 90% of patients that is often refractory to surgical or targeted pharmacological intervention. In addition to seizures, TSC-associated neurological disorders (TANDs) are present in up to 85% and intellectual disability is present in up to 70% of patients. Interestingly, single TSC1 or TSC2 mutations can have differential effects between affected individuals, and even identical inherited variants can result in different disease profiles. This indicates significant heterogeneity in patient outcomes, the mechanisms for which is currently unknown; understanding the mechanism for this difference in prognosis will allow us to identify novel therapeutic approaches capable of modifying outcome in TSC.
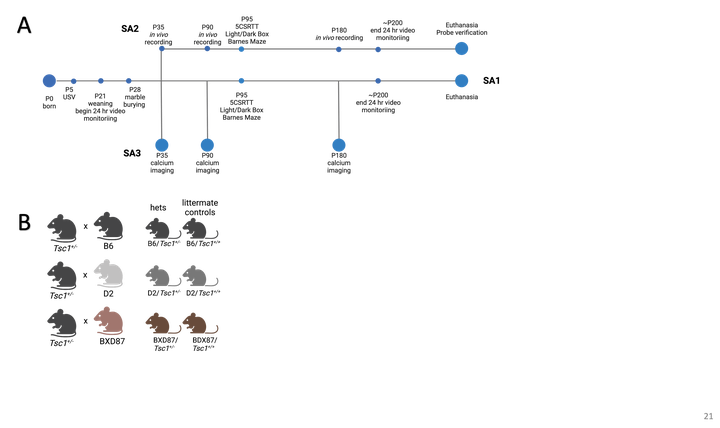
We recently developed and validated a model system of this heterogeneity to improve our ability to probe the basic biology of TSC and explore the neural network differences underlying seizures and TANDs. Our novel mice have a germline Tsc1 heterozygous knockout which, when introduced to controlled genetic background diversity, faithfully recapitulate patient heterogeneity in TSC outcomes with the clinically relevant gene dosage and expression pattern. We have remarkable preliminary data showing that the same Tsc1 haploinsufficiency results in a spectrum of background-dependent differences in TSC outcomes. This spectrum allows us to explore how networks underlying cognition and seizure are differentially affected by the same precipitating genetic insult. We hypothesize that genetic background-associated differences in seizure and TANDs outcomes resulting from Tsc1 haploinsufficiency will manifest as changes in neuronal dynamics, partially mediated by aberrant astrocyte modulation of these neuronal dynamics.
We will test this hypothesis with the following aims: 1) Phenotype the repertoire of behavioral impairments in Tsc1 haploinsufficient mice, 2) Characterize the relationship between changes in neuronal dynamics, seizure and TANDs, 3) Describe changes in excitatory/inhibitory dynamics and explore the role of non-neuronal populations in changes in E/I balance that underlie epilepsy.